Analysis of Antimony Removal with Modified Activated Carbon Using Response Surface Methodology
Abstract
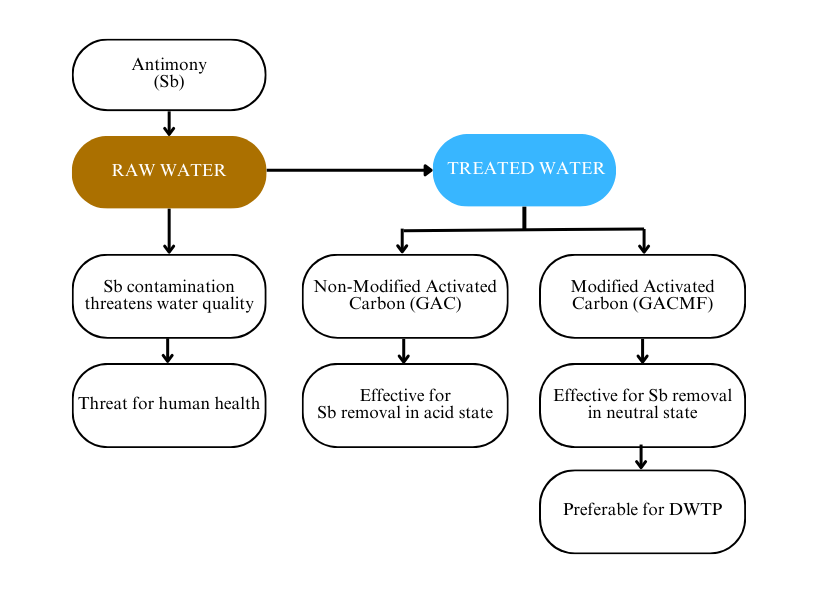
Antimony (Sb) is a metal compound that can cause health problems when it accumulates in the food chain and becomes toxic to the ecosystem. Industrial activities have driven Sb contamination of water, including surface water. At the same time, some drinking water treatment plants (DWTP) use surface water as raw water, which requires adequate treatment. An invention of cheap and accessible technology is needed for developing countries such as Indonesia; hence, this study presents research on modified activated carbon with iron sulfate and manganese sulfate to create a better adsorbent from commercial granular activated carbon (GAC). The independent variables in this study included the type of adsorbent (GAC and GACMF), acidity level, and dosage. Response surface methodology was implemented for the analysis. According to the study, it was found that the optimum state of non-modified GAC for Sb removal appears at pH 3 and a dosage of 0.03 g L-1. In contrast, the presence of modified GAC was more effective for Sb removal with an optimum pH of 6 and a dosage of 0.057 g L-1 for GACMF. This research suggests that GACMF is preferable for DWTP because the optimization shows that GACMF is optimized in a neutral state; therefore, additional chemicals are unnecessary to achieve a neutral acidity state.
References
Bolan, N., Kumar, M., Singh, E., Kumar, A., Singh, L., Kumar, S., Keerthanan, S., Hoang, S. A., El-Naggar, A., Vithanage, M., Sarkar, B., Wijesekara, H., Diyabalanage, S., Sooriyakumar, P., Vinu, A., Wang, H., Kirkham, M. B., Shaheen, S. M., Rinklebe, J., & Siddique, K. H. M. 2022. Antimony contamination and its risk management in complex environmental settings: A review. Environment International, 158. https://doi.org/10.1016/j.envint.2021.106908.
Eppinger, R.G., Briggs, P.H., Crock, J.G., Meier, A.L., Sutley, S.J., Theodorakos, P.M., 2000. Environmental–geochemical study of the Slate Creek antimony deposit, Kantishna Hills, Denali National Park and Preserve, Alaska. Studies by the U.S. Geological Survey in Alaska, 2000: U.S Geological Survey Professional Paper, 1662. https://pubs.usgs.gov/pp/pp1662/chap8.pdf [Online Accessed: June 14, 2023, at 23.50 (GMT +7)].
Filella, M., Hennebert, P., Okkenhaug, G., & Turner, A. 2020. Occurrence and fate of antimony in plastics. Journal of Hazardous Materials, 390. https://doi.org/10.1016/j.jhazmat.2019.121764.
IKPLHD (Informasi Kinerja Pengelolaan Lingkungan Hidup) Kota Surabaya. 2020. Available online: https://lh.surabaya.go.id/fileupload/ebook/IKPLHD%20KOTA%20SURABAYA%202020%20FINAL.pdf.
Ilavský, J. 2008. Removal of Antimony from Water by Sorption Materials. Slovak Journal of Civil Engineering 2:1-6.
Indonesian Government. 2010. Minister of Health No. 492 of 2010 about the drinking water requirement. Jakarta.
Indonesian Government. 2013. Governor of East Java No. 32 of 2013 about Wastewater Quality Standards. Surabaya.
Lai, Z., He, M., Lin, C., Ouyang, W., & Liu, X. 2022. Interactions of antimony with biomolecules and its effects on human health. In Ecotoxicology and Environmental Safety (Vol. 233). Academic Press. https://doi.org/10.1016/j.ecoenv.2022.113317.
Li, J, BoHong Zheng, Yangzhuo He, Yaoyu Zhou, Xiao Chen, Shan Ruan, Yuan Yang, Chunhao Dai, Lin Tang. 2018. Antimony contamination, consequences and removal techniques: A review. Ecotoxicology and Environmental Safety, 156, 125–134. https://doi.org/10.1016/j.ecoenv.2018.03.024.
Liu, H., Ying, Q., Li, C., Norra, S., & Lichtfouse, E. 2020. Enhanced removal of antimony in dyeing wastewater by mixing Fe3O4 with manganese sand filter material. Water Environment Research, 92(8), 1208–1213. https://doi.org/10.1002/wer.1315.
Liu, R., Liu, F., Hu, C., He, Z., Liu, H., & Qu, J. 2015. Simultaneous removal of Cd(II) and Sb(V) by Fe-Mn binary oxide: Positive effects of Cd(II) on Sb(V) adsorption. Journal of Hazardous Materials, 300, 847–854. https://doi.org/10.1016/j.jhazmat.2015.08.020.
Liu, R., Xu, W., He, Z., Lan, H., Liu, H., Qu, J., & Prasai, T. 2015. Adsorption of antimony(V) onto Mn(II)-enriched surfaces of manganese-oxide and FeMn binary oxide. Chemosphere, 138: 616–624. https://doi.org/10.1016/j.chemosphere.2015.07.039.
Long, X., Wang, X., Guo, X., & He, M. 2020. A review of removal technology for antimony in aqueous solution. In Journal of Environmental Sciences (China) 90: 189–204. Chinese Academy of Sciences. https://doi.org/10.1016/j.jes.2019.12.008.
Nishad, P. A., & Bhaskarapillai, A. 2021. Antimony, a pollutant of emerging concern: A review on industrial sources and remediation technologies. In Chemosphere 277. Elsevier Ltd. https://doi.org/10.1016/j.chemosphere.2021.130252.
Nurdin, Fahmi A., et al. 2014. “Surabaya Underground Aqua Project” Konsep Pengelolaan Air Minum, Air Limbah, dan Air Hujan Perkotaan di Bawah Tanah sebagai Solusi Permasalahan Air di Kota Besar." Pekan Ilmiah Mahasiswa Nasional Program Kreativitas Mahasiswa - Gagasan Tertulis 2014, Jakarta, Indonesia, 2014. Indonesian Ministry of Research, Technology and Higher Education. Available Onling: https://media.neliti.com/media/publications/170100-ID-surabaya-underground-aqua-project-konsep.pdf
Pintor, A.M.A., Vieira, B.R.C., Boaventura, R.A.R., & Botelho, C.M.S. 2020. Removal of Antimony from water by iron-coated cork granulates. Separation and Purification Technology 233: 116020. https://doi.org/10.1016/j.seppur.2019.116020.
Roh, J. S., Park, J. S., Roh, J. M., Park, H. B., & Do, S. H. 2018. The pretreatment of granular activated carbon using sodium persulfate and hydrogen peroxide under basic conditions: Properties, metal impregnation, and As(V) adsorption. Materials Chemistry and Physics, 218: 317–325. https://doi.org/10.1016/j.matchemphys.2018.07.045.
Qiao, D. 2016. Public Health Goal for ANTIMONY in Drinking Water. [Internet]. Available online: https://oehha.ca.gov/media/downloads/water/chemicals/phg/antimonyphg092316.pdf
Ungureanu, G., Santos, S., Boaventura, R., & Botelho, C. 2015. Arsenic and antimony in water and wastewater: Overview of removal techniques with special reference to latest advances in adsorption. Journal of Environmental Management 151: 326–342. Academic Press. https://doi.org/10.1016/j.jenvman.2014.12.051.
Wan, S, Lian Qiu, Yan Li, Junjie Sun, Bin Gao, Feng He, Wubo Wan. 2022. Accelerated antimony and copper removal by manganese oxide embedded in biochar with enlarged pore structure. Chemical Engineering Journal Vol. 404: 126021 https://doi.org/10.1016/j.cej.2020.126021
Yu, T., Wang, X., & Li, C. 2014. Removal of Antimony by FeCl3-Modified Granular-Activated Carbon in Aqueous Solution. Journal of Environmental Engineering, 140(9). https://doi.org/10.1061/(asce)ee.1943-7870.0000736.
Yudo, S, and Nusa I.S. 2019. Kondisi Kualitas Air Sungai Surabaya Studi Kasus: Peningkatan Kualitas Air Baku PDAM Surabaya. Jurnal Teknologi Lingkungan 20(1): 19-28. https://doi.org/10.29122/jtl.v20i1.2547.
Zhang, X., Xie, N., Guo, Y., Niu, D., Sun, H., Yang, Y. 2020. Insight into adsorptive removal of antimony contaminants: Functional materials, evaluation and prospective. Journal of Hazardous Materials, 418(126345). https://doi.org/10.1016/j.jhamzat.2021.126345.
Authors

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access).